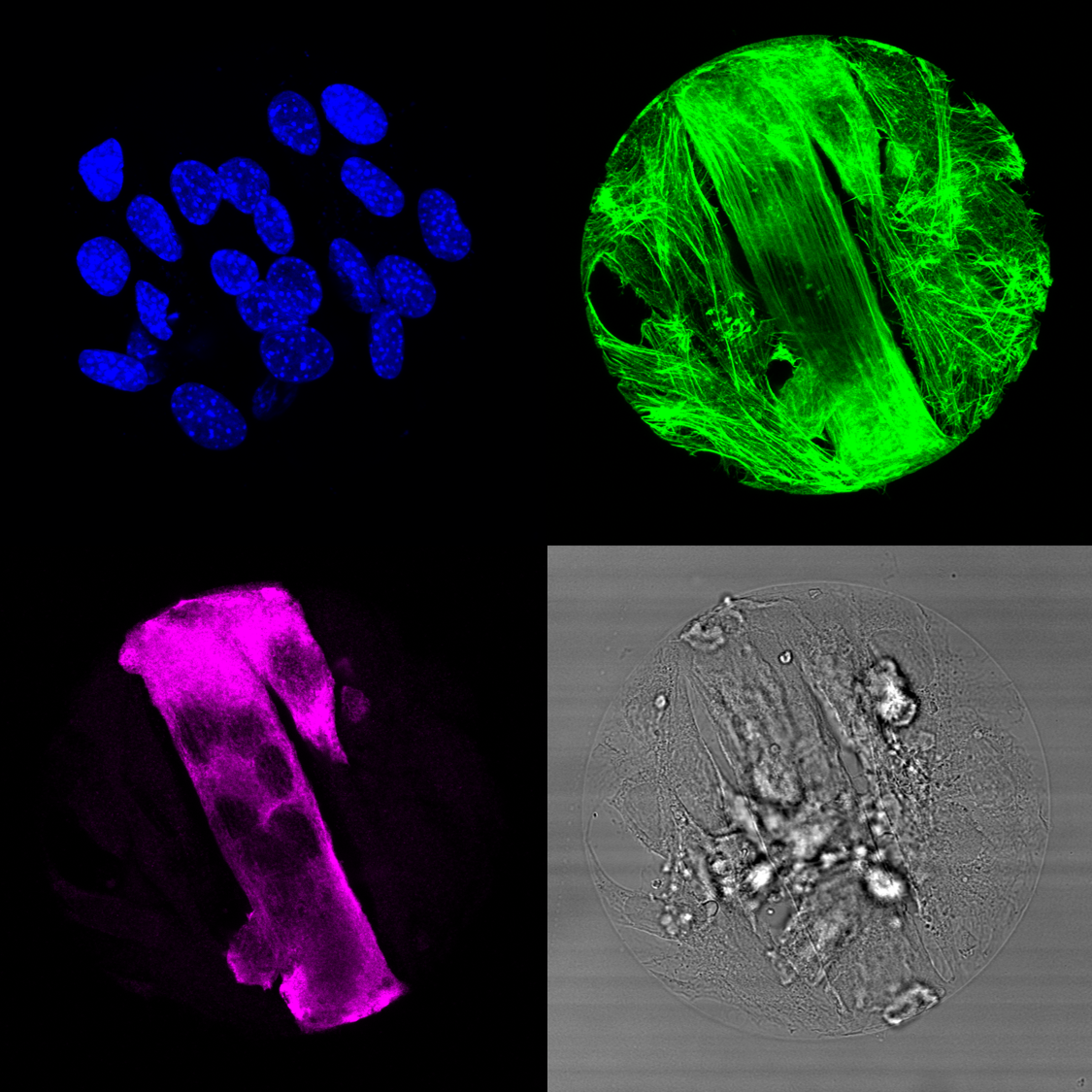
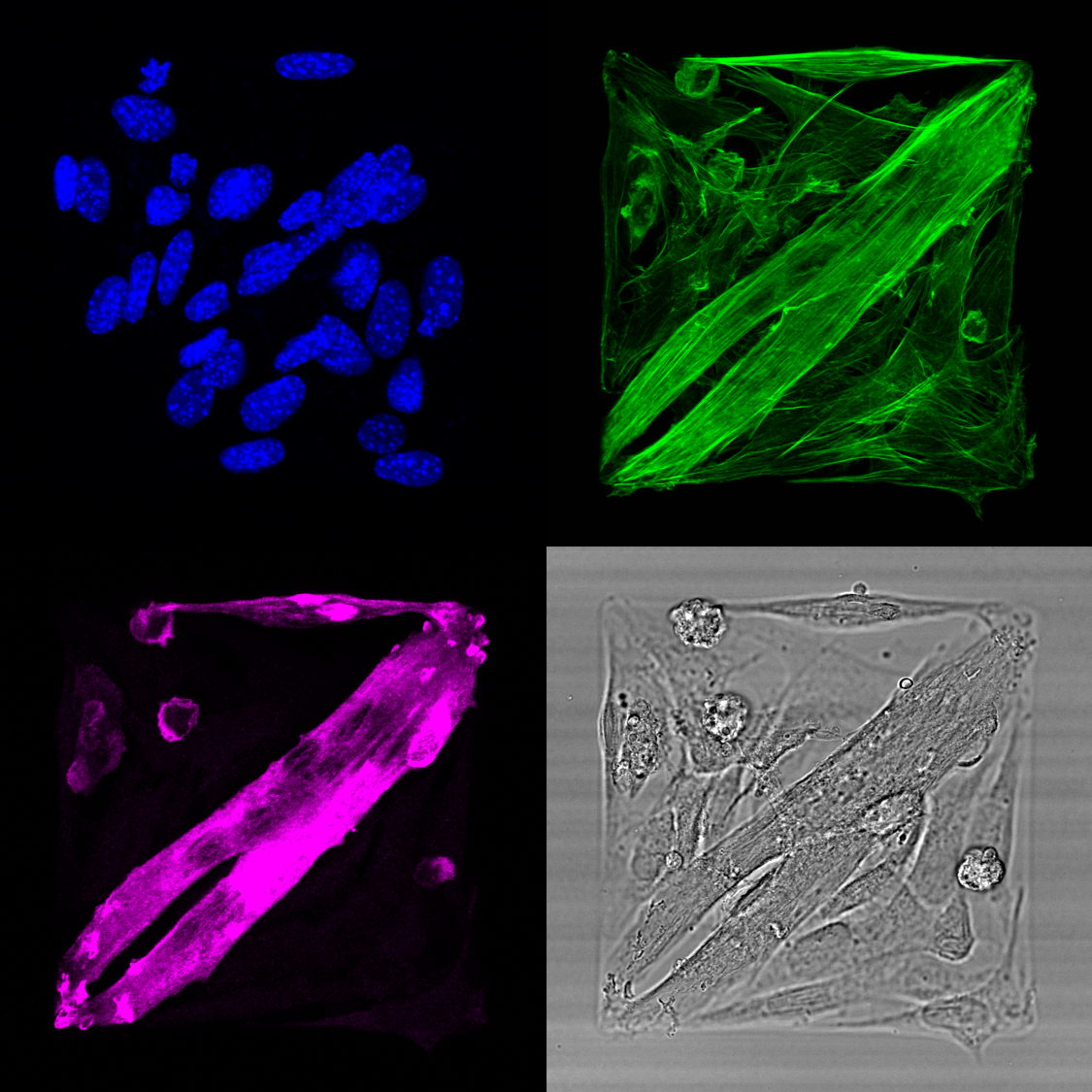
Major insights into the mechanics of cell-cell fusion have been revealed by studies in Drosophila. During Drosophila myogenesis, mononucleated muscle cells (Myoblasts) fuse to form multinucleated muscle fibers. Myoblast fusion is an asymmetric process in which one cell invades its partner using actin-propelled membrane protrusions to promote fusion. Similar protrusions have since been observed by electron microscopy (EM) studies of mammalian cells, suggesting that these invasive protrusions could be a conserved mechanism. However, no live imaging analyses have been performed to demonstrate that these invasive protrusions correspond to sites of fusion. Moreover, it is unclear whether the recently identified mammalian myoblast fusogenic proteins (Myomaker and Myomixer) are localized to these protrusions. To answer these questions, we have developed micropattern approach to observe the dynamics of the myoblast cell-cell fusion.
2024
- Lu Y. et al. – Molecular Regulation of Invasive Protrusion Formation at the Mammalian Fusogenic Synapse, bioRxiv.
Invasive membrane protrusions play a central role in a variety of cellular processes. Unlike filopodia, invasive protrusions are mechanically stiff and propelled by branched actin polymerization. However, how branched actin filaments are organized to create finger-like invasive protrusions remains a longstanding question in cell biology. Here, by examining the mammalian fusogenic synapse, where invasive protrusions are generated to promote cell membrane juxtaposition and fusion, we have uncovered the mechanism underlying invasive protrusion formation. We show that two Arp2/3 nucleation promoting factors (NPFs), WAVE and N-WASP, exhibit distinct and complementary localization patterns in the protrusions. While WAVE is at the leading edge, N-WASP is recruited by its interacting protein, WIP, to the shaft of the protrusion. During protrusion growth, new branched actin filaments are polymerized at the periphery of the shaft and crosslinked to preexisting actin bundles by the “pioneer” actin-bundling protein dynamin. The thickened actin bundles are further stabilized by WIP, which functions as a WH2 domain-mediated actin-bundling protein. Disrupting any of these components results in defective protrusions and myoblast fusion in cultured cells and/or in mouse embryos. Thus, our study has revealed the intricate spatiotemporal coordination between two NPFs and two actin-bundling proteins in creating invasive protrusions and has general implications in understanding protrusion formation in many cellular processes beyond cell-cell fusion.
Talks and Poster Presentation
2022
- Written communication at the National Postdoc Appreciation Week, Dallas, Texas.
2021
- Selected speaker at the Regenerative Science and Medicine meeting: « Are actin-propelled membrane protrusions involved in the mammalian myoblast fusion? », Dallas, Texas.
2020
- Selected speaker at the Muscular Dystrophy Cooperative Research meeting: « The role of YAP in skeletal muscle differentiation and Duchenne muscular dystrophy », Dallas, Texas.
